EDITORS NOTE: I (Will) recently did a video on ARA which discusses a recent study that found ARA had positive effects on strength and muscle mass readers will want to check out. The results of this study will be covered HERE and in a future article by Monica. This excellent article below by Monica discusses the safety of ARA supplements and possible health benefits that set the record straight on this fatty acid…
In part 1 I outlined the background to the “ARA is bad” theory, and presented studies that have refuted this notion. Part 1 also explains the importance of distinguishing the different omega-6 fatty acids, LA and ARA, and describes the bell-shaped relationship between ARA and EPA + DHA in cell membranes.
In this part you will learn about safety aspects and potential health benefits (!) of ARA supplementation…
Safety and Health Effects of ARA supplementation
With the bad reputation that ARA has, let’s start by looking at safety data. On a typical modern diet (that includes meat, eggs and fish) the average intake of ARA is approximately 100–200 mg ARA per day.[1-5] Several studies have investigated safety aspects of ARA supplementation in different populations.
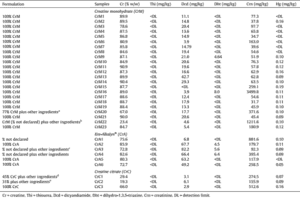
When healthy volunteers were given over 7 times the usual intake of ARA (i.e. 1500 to 1700 g ARA per day, compared to usual intake of 200 mg ARA per day) in a 7 week controlled feeding study, no effects on platelet aggregation, bleeding times, the balance of vasoactive metabolites, serum lipid levels, or immune response were observed.[6-10] Likewise, in a recent study on healthy men aged 26-60 years, supplementation with 840 mg ARA per day for 4 weeks had no effect on any metabolic parameter or platelet function.[11]
A study in healthy Japanese men and women aged 55-70 investigated whether ARA supplementation affects clinical parameters involved in cardiovascular, inflammatory, and allergic diseases.[12] Subjects were supplemented with ARA-enriched oil (240 or 720 mg ARA per day) or placebo for 4 weeks, followed by a 4-week washout period. The fatty acid contents of plasma phospholipids, clinical parameters, and AA metabolites were determined at baseline, 2, 4, and 8 weeks. It was found that ARA content in plasma phospholipids in the ARA supplemented groups increased dose-dependently and was almost the same at 2 weeks and at 4 weeks. The elevated ARA content decreased to nearly baseline during a 4-week washout period. Contrary to expectations, during the supplementation and washout periods, no changes were observed in plasma phospholipid EPA and DHA content. There were no changes in clinical blood parameters related to cardiovascular, inflammatory and allergic diseases.[12]
Can ARA supplementation possibly have beneficial health promoting effects?
While ARA is most known for giving rise to pro-inflammatory, vasoconstrictive, and/or pro-aggregatory eicosanoids (such as PGE2, thromboxane A2, and leukotriene B4), ARA also produces eicosanoids that are anti-inflammatory and/or anti-aggregatory (such as prostacyclin, lipoxin A4 [13, 14] and epoxyeicosatrienoic acids [15]).
Thus, the ARA-derived prostaglandin PGE2 is not a clear-cut pro-inflammatory “devil”.[16] More specifically, PGE2 also inhibits production of some inflammatory cytokines TNF-alpha and IL-1 in immune cells (macrophages and monocytes)[17] and inflammatory leukotrienes [18], and induces production of inflammation resolving lipoxin A4. 74, 75
As these studies demonstrate, PGE2 from ARA acts as both a pro- and anti-inflammatory agent, and may be responsible for helping to turn off and “resolve” inflammation through the inhibition of 5-lipoxygenase and the production of lipoxins.[19] There is accumulating evidence that lipoxins are promising therapeutic agents for inflammatory disorders leading to tissue damage.[20] It is notable that ARA supplementation dose-dependently increases production of lipoxin A4.[21] Further, it is possible that the pro-resolving effect of ARA-derived eicosanoids may work in concert with the resolvins from EPA and DHA, and protectins from DHA.[22]
Because resolution of inflammation is a distinct process from anti-inflammatory processes [23], and because ARA gives rise to both inflammatory/anti-inflammatory and pro-resolving lipid mediators, the statement that ARA is “bad” due its involvement in the initiation of inflammation is a flawed over-simplification that is blatantly incorrect. Also this data indicated that the anti-inflammatory effects of dietary fish oil may not be mediated through a simple substitution of one family of eicosanoids for another.[17]
A Science Advisory from the American Heart Association (AHA) acknowledged an additional potentially beneficial role of ARA [24]; it’s conversion to epoxy-eicosatrienoic acids by cytochrome P450.[15] These compounds are fatty acid epoxides that have potent vasodilator properties by inducing relaxation of vascular smooth muscle cells.[25]
ARA supplementation is gaining increasing attention. An experimental study showed that ARA supplementation ameliorates diabetes [26], and it has been suggested that ARA acts as an anti-diabetic molecule [27]. Thus, ARA supplementation may possibly help prevent development of diabetes and it consequences in high-risk populations.[26, 27] In addition, ARA supplementation has been shown to significantly protect against the teratogenic action of high blood sugar levels (hyperglycemia).[28]
It has been demonstrated that people over 56 years of age, compared with young adults in their 20s, have a lower amount of ARA in red blood cell phospholipids, after controlling for the potential influence EPA+DHA intake.[29] One study found in healthy elderly men that supplementing with 600 mg ARA per day for 1 month not only significantly increased in ARA content in serum phospholipids, but also improved measures of cognitive function compared with olive oil placebo.[30] This is in line with findings from animal studies. In aged rats, brain levels of ARA and DHA are depleted and memory is impaired (measured by long-term potentiation, the neurophysiological correlate of memory), and restoring brain ARA levels was found to improve memory.[31]
Another study supplemented Japanese elderly individuals aged over 65 years with 240 mg ARA per day + 240 mg DHA per day for 3 months, and demonstrated improved blood flow in the microcirculation of the heart muscle.[32] Thus, ARA and DHA may be beneficial in preventing and/or improving age-related declines in brain and cardiovascular function.[33] This is supported by findings that EPA, DHA, and ARA all may be of benefit in dementia and Alzheimer’s disease by up-regulating gene expression related to neurogenesis, improving neurotransmission and nerve cell communication, elevating endothelial nitric oxide (eNO) production and enhancing brain levels of the neurotransmitter acetylcholine.[34]
Experimental data indicates that ARA or PGE2 prevents gene expression of lipogenic (fat synthesizing) genes in the liver and fat cells.[35, 36] A recent intriguing experimental study showed that ARA, or some of its lipid metabolites, promotes resistance to starvation by acting as signals of reduced food availability, and consequently trigger a fasting program that extends life span, even in an abundant food regimen.[37] It is especially notable that elevated levels of ARA, but not EPA, activate autophagy, which is a physiological process that promotes life span extension by clearing cellular damage.[38-41] By showing that AA can activate autophagy and extend longevity without caloric restriction, these study authors argue that increasing levels of ARA may contribute to life span extension.[37]
What about the omega-6/omega-3 ratio?
Since ARA is an omega-3 fatty acid, you may wonder if supplementing with it might screw up the omega-6/omega-3 ratio in the diet by elevating it. There answer is no. Even when supplementing with 1.5 g ARA per day, this won’t add much to the omega-6 load in your diet. Most of the omega-6 we ingest comes from LA. Typical LA intakes in US adults ranges from approximately 12 to 17 g/d for men and 9 to 11 g/d for women (in contrast, the total n-3 fatty intake for men and women is only 1.3 to 1.8 g/d and 1.0 to 1.2 g/d, respectively) [42], which is about ten times the amount of ARA you would get from an effective ARA supplementation dose.
Even more importantly, the omega-6/omega-3 ratio is futile. The ratio of omega-6/omega-3 fats in most modern diets is in excess of 10:1, and a ratio of 4:1 or lower has been suggested as optimal for health promotion and disease prevention.[43-48] The suggestion of an optimal omega-6/omega-3 ratio is based on old studies suggesting that high intakes of LA lead to reduced conversion of ALA to EPA due to competition between LA and ALA for the enzyme (delta-6 desaturase) that converts these fatty acids to their respective longer-chain fatty acids (ARA and EPA), resulting in increased tissue ARA levels and reduced EPA and DHA levels.[43, 45, 49] Support for the hypothesis that a high intake of LA inhibits the conversion of ALA to EPA comes from a study by showing that a dietary LA/ALA ratio (omega-6/omega-3 ratio) of 2:1 [50] or 4:1 [51] as compared with 10:1 results in a higher plasma phospholipid EPA levels. But because the conversion of ALA to EPA and especially DHA is so inefficient even at low omega-6/omega-3 ratios and high ALA intakes [50, 52-54], this argument is moot. For example, with a background diet high in saturated fat conversion ALA is approximately 6% for EPA [52]. With a diet rich in n-6 PUFA, conversion is reduced by 40 to 50%.[52] Even at a 6% conversion rate, to achieve an effective dose of EPA (e.g. 2.5 g), one would have to consume over 40 g of ALA! That would require 6 tbsp flaxseed oil or 16 oz (450 g) walnuts (7.2 g ALA per tbsp flaxseed oil, 2.5 g ALA per 28.35 g walnuts).[55] And you’d be lucky if your body produces any significant amount of DHA out of that mega load![50, 54]
Another problem with the omega-6/omega-3 is that it does not distinguish between the different omega-6 (LA, GLA, ARA) and omega-3 fatty acids (ALA, EPA, DHA), which all have different effects (albeit sometimes overlapping), and the ratio can be changed in many different ways [56-68]. Also, the omega-6/omega-3 does not say anything about absolute tissue levels of each individual omega-6 and omega-3 fatty acids, which is likely the factor that matters the most.[68, 69] Therefore, comprehensive reviews by leading nutrition researchers have concluded that the omega-6/omega-3 ratio is misleading and not useful.[69, 70] Hence, it makes more sense to look at the ARA/EPA ratio (or the ARA/EPA+DHA ratio), and especially their absolute tissue levels.[71, 72]
Should I supplement with both ARA and fish oil?
As stated above, ARA supplementation does not affect cell membrane phospholipid levels of EPA or DHA.[9, 11, 12, 73] However, fish oil (both EPA and DHA) reduces blood ARA levels and down-regulates ARA incorporation into tissue membrane phospholipids.[61, 74-76] This is in line with the bell-shaped relationship between ARA and EPA + DHA in cell membranes. Therefore, if you are taking a high-dose fish oil, your tissue levels of ARA may actually be below optimal. Therefore, co-supplementing with ARA and fish oil is a good idea and strongly recommended.
On the other hand, supplementing with ARA without fish oil may raise ARA levels too much. This could potentially have a negative effect in the brain, as ARA might increase production of A-beta amyloid [77], the molecular key event triggering the development and progress of Alzheimer’s Disease.[78-80] This potential side-effect of too much ARA may be counteracted by DHA (found in fish oil and algae oil), as DHA lowers A-beta amyloid levels.[77, 81]
One study showed that supplementing with an oil blend providing a high daily dose of 3600 mg ARA and 2900 mg DHA for 14 days is safe in healthy men aged 19-39 years is safe.[82] This 1.25:1 ratio is similar to the ARA:DHA ratio in human milk [83], and the study researchers suggested that the safety of this high dose of ARA was due to it being ingested together with DHA. [82] Thus, when supplementing with 1500 mg ARA/day with the goal to enhance muscle growth, strength gains and performance (more on that in part 3), it is recommended that you also supplement with fish oil providing a least 1200 mg DHA (you want to make sure you get your EPA also).
Conclusion
ARA supplementation of up to 1.5 g per day is safe for people with no pre-existing inflammatory conditions.
Important take-homes from part 1 and part 2 of this series are:
* ARA is not a universally “bad” fatty acid as previously thought. To the contrary, low ARA levels in tissue membranes have been linked to negative health outcomes.
* Contrary to entrenched belief, you cannot rely on your intake of LA (linoleic acid, the parent omega-6 fatty acid) to get enough ARA, because the conversion of LA to ARA is minimal.
* Always supplement ARA together with fish oil. Higher blood levels of both ARA and omega-3 fatty acids are associated with the lowest level of inflammatory markers.[84, 85] A safe and effective dose of ARA is 1.5 g per day. Combine this with a fish oil supplement providing around 2.5 g EPA and 2.5 g DHA per day.
* Optimal health requires raising the levels of both ARA and EPA+DHA in tissues, and not either one at the expense of the other [86-90] and it is low levels of these three main essential fatty acids that cause harm. [86, 91-97] When it comes to ARA (omega-6) and EPA+DHA (omega-3) the key is collaboration and not antagonism.[87] A good example of this is that ARA has a U-shaped relationship with the risk of heart attack; both too low and to high ARA levels in cell membranes are associated with higher heart attack risk.[98, 99]
* Supplementing with both ARA and fish oil (EPA + DHA) together will provide the best of both worlds because intake of ARA is need to enrich cell membranes with ARA, while intake of EPA + DHA will ensure that the cell membranes level of ARA stays in the healthy range (thanks to the bell-shaped relation between these fatty acids, as outlined above).[100] An omega-3 index (level of EPA+DHA in red blood cells) of 8-11% (which is recommended for cardiovascular health promotion[101-103]) coincides with an ARA level in red blood cell of about 14-15% [100] (the bottom of the risk U-shape relationship) which is the most protective ARA level for heart disease.[98]
Coming next in part 3…
Previous studies have shown that supplementing with EPA + DHA from fish oil increases muscle protein synthesis. [104, 105] Two studies have been conducted on ARA supplementation, which specifically looked at performance enhancing effects. The latest ARA supplementation study also investigated potential muscle growth stimulating effects of ARA… This will be the topic of part 3 of this series…stay tuned!
EFFECTS OF ARA ON STRENGTH AND MUSCLE MASS COVERED HERE
References:
1. Kawabata, T., et al., Age-related changes of dietary intake and blood eicosapentaenoic acid, docosahexaenoic acid, and arachidonic acid levels in Japanese men and women. Prostaglandins Leukot Essent Fatty Acids, 2011. 84(5-6): p. 131-7.
2. Linseisen, J., et al., Quantity and quality of dietary fat, carbohydrate, and fiber intake in the German EPIC cohorts. Ann Nutr Metab, 2003. 47(1): p. 37-46.
3. Sioen, I.A., et al., Dietary intakes and food sources of fatty acids for Belgian women, focused on n-6 and n-3 polyunsaturated fatty acids. Lipids, 2006. 41(5): p. 415-22.
4. Mann, N.J., et al., The arachidonic acid content of the Australian diet is lower than previously estimated. J Nutr, 1995. 125(10): p. 2528-35.
5. Farina, E.K., et al., Dietary intakes of arachidonic acid and alpha-linolenic acid are associated with reduced risk of hip fracture in older adults. J Nutr, 2011. 141(6): p. 1146-53.
6. Ferretti, A., et al., Increased dietary arachidonic acid enhances the synthesis of vasoactive eicosanoids in humans. Lipids, 1997. 32(4): p. 435-9.
7. Nelson, G.J., et al., The effect of dietary arachidonic acid on plasma lipoprotein distributions, apoproteins, blood lipid levels, and tissue fatty acid composition in humans. Lipids, 1997. 32(4): p. 427-33.
8. Kelley, D.S., et al., Effects of dietary arachidonic acid on human immune response. Lipids, 1997. 32(4): p. 449-56.
9. Nelson, G.J., et al., The effect of dietary arachidonic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans. Lipids, 1997. 32(4): p. 421-5.
10. Kelley, D.S., et al., Arachidonic acid supplementation enhances synthesis of eicosanoids without suppressing immune functions in young healthy men. Lipids, 1998. 33(2): p. 125-30.
11. Kusumoto, A., et al., Effects of arachidonate-enriched triacylglycerol supplementation on serum fatty acids and platelet aggregation in healthy male subjects with a fish diet. Br J Nutr, 2007. 98(3): p. 626-35.
12. Kakutani, S., et al., Supplementation of arachidonic acid-enriched oil increases arachidonic acid contents in plasma phospholipids, but does not increase their metabolites and clinical parameters in Japanese healthy elderly individuals: a randomized controlled study. Lipids Health Dis, 2011. 10: p. 241.
13. Chiang, N., M. Arita, and C.N. Serhan, Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids, 2005. 73(3-4): p. 163-77.
14. Serhan, C.N., Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids, 2005. 73(3-4): p. 141-62.
15. Node, K., et al., Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science, 1999. 285(5431): p. 1276-9.
16. Calder, P.C., Polyunsaturated fatty acids and inflammatory processes: New twists in an old tale. Biochimie, 2009. 91(6): p. 791-5.
17. Miles, E.A., E. Allen, and P.C. Calder, In vitro effects of eicosanoids derived from different 20-carbon Fatty acids on production of monocyte-derived cytokines in human whole blood cultures. Cytokine, 2002. 20(5): p. 215-23.
18. Levy, B.D., et al., Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol, 2001. 2(7): p. 612-9.
19. Serhan, C.N., Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol, 2007. 25: p. 101-37.
20. Ryan, A. and C. Godson, Lipoxins: regulators of resolution. Curr Opin Pharmacol, 2010. 10(2): p. 166-72.
21. Tateishi, N., et al., Dietary supplementation of arachidonic acid increases arachidonic acid and lipoxin A4 contents in colon, but does not affect severity or prostaglandin E2 content in murine colitis model. Lipids Health Dis, 2014. 13(1): p. 30.
22. Serhan, C.N. and N.A. Petasis, Resolvins and protectins in inflammation resolution. Chem Rev, 2011. 111(10): p. 5922-43.
23. Serhan, C.N., et al., Resolution of inflammation: state of the art, definitions and terms. FASEB J, 2007. 21(2): p. 325-32.
24. Harris, W.S., et al., Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation, 2009. 119(6): p. 902-7.
25. Oltman, C.L., et al., Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res, 1998. 83(9): p. 932-9.
26. Suresh, Y. and U.N. Das, Protective action of arachidonic acid against alloxan-induced cytotoxicity and diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids, 2001. 64(1): p. 37-52.
27. Das, U.N., Arachidonic acid and lipoxin A4 as possible endogenous anti-diabetic molecules. Prostaglandins Leukot Essent Fatty Acids, 2013. 88(3): p. 201-10.
28. Goldman, A.S., et al., Hyperglycemia-induced teratogenesis is mediated by a functional deficiency of arachidonic acid. Proc Natl Acad Sci U S A, 1985. 82(23): p. 8227-31.
29. Kawabata, T., et al., Associations between dietary n-6 and n-3 fatty acids and arachidonic acid compositions in plasma and erythrocytes in young and elderly Japanese volunteers. Lipids Health Dis, 2011. 10: p. 138.
30. Ishikura, Y., et al., Arachidonic acid supplementation decreases P300 latency and increases P300 amplitude of event-related potentials in healthy elderly men. Neuropsychobiology, 2009. 60(2): p. 73-9.
31. Martin, D.S., et al., Long-term potentiation in aged rats is restored when the age-related decrease in polyunsaturated fatty acid concentration is reversed. Prostaglandins Leukot Essent Fatty Acids, 2002. 67(2-3): p. 121-30.
32. Oe, H., et al., Arachidonic acid and docosahexaenoic acid supplementation increases coronary flow velocity reserve in Japanese elderly individuals. Heart, 2008. 94(3): p. 316-21.
33. Kiso, Y., Pharmacology in health foods: effects of arachidonic acid and docosahexaenoic acid on the age-related decline in brain and cardiovascular system function. J Pharmacol Sci, 2011. 115(4): p. 471-5.
34. Das, U.N., Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer’s disease–but how and why? Prostaglandins Leukot Essent Fatty Acids, 2008. 78(1): p. 11-9.
35. Mater, M.K., A.P. Thelen, and D.B. Jump, Arachidonic acid and PGE2 regulation of hepatic lipogenic gene expression. J Lipid Res, 1999. 40(6): p. 1045-52.
36. Oikawa, D., et al., Arachidonic acid prevents fatty liver induced by conjugated linoleic acid in mice. Br J Nutr, 2009. 101(10): p. 1558-63.
37. O’Rourke, E.J., et al., omega-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Genes Dev, 2013. 27(4): p. 429-40.
38. Cuervo, A.M., Autophagy and aging: keeping that old broom working. Trends Genet, 2008. 24(12): p. 604-12.
39. Madeo, F., N. Tavernarakis, and G. Kroemer, Can autophagy promote longevity? Nat Cell Biol, 2010. 12(9): p. 842-6.
40. Rabinowitz, J.D. and E. White, Autophagy and metabolism. Science, 2010. 330(6009): p. 1344-8.
41. Rubinsztein, D.C., G. Marino, and G. Kroemer, Autophagy and aging. Cell, 2011. 146(5): p. 682-95.
42. Food and Nutrition Board (FNB) and Institute of Medicine (IOM), Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids, 2005: National Academies Press (October 28, 2005).
43. Kris-Etherton, P.M., K.D. Hecker, and A.E. Binkoski, Polyunsaturated fatty acids and cardiovascular health. Nutr Rev, 2004. 62(11): p. 414-26.
44. Simopoulos, A.P., Commentary on the workshop statement. Essentiality of and recommended dietary intakes for Omega-6 and Omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids, 2000. 63(3): p. 123-4.
45. Simopoulos, A.P., n-3 fatty acids and human health: defining strategies for public policy. Lipids, 2001. 36 Suppl: p. S83-9.
46. Simopoulos, A.P., The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother, 2002. 56(8): p. 365-79.
47. Simopoulos, A.P., Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother, 2006. 60(9): p. 502-7.
48. Simopoulos, A.P., A. Leaf, and N. Salem, Jr., Workshop statement on the essentiality of and recommended dietary intakes for Omega-6 and Omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids, 2000. 63(3): p. 119-21.
49. Chan, J.K., et al., Effect of dietary alpha-linolenic acid and its ratio to linoleic acid on platelet and plasma fatty acids and thrombogenesis. Lipids, 1993. 28(9): p. 811-7.
50. Wien, M., et al., Decreasing the linoleic acid to alpha-linolenic acid diet ratio increases eicosapentaenoic acid in erythrocytes in adults. Lipids, 2010. 45(8): p. 683-92.
51. Liou, Y.A., et al., Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr, 2007. 137(4): p. 945-52.
52. Gerster, H., Can adults adequately convert alpha-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? Int J Vitam Nutr Res, 1998. 68(3): p. 159-73.
53. Brenna, J.T., et al., alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids, 2009. 80(2-3): p. 85-91.
54. Arterburn, L.M., E.B. Hall, and H. Oken, Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr, 2006. 83(6 Suppl): p. 1467S-1476S.
55. Gebauer, S.K., et al., n-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am J Clin Nutr, 2006. 83(6 Suppl): p. 1526S-1535S.
56. Anderson, B.M. and D.W. Ma, Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis, 2009. 8: p. 33.
57. Cottin, S.C., T.A. Sanders, and W.L. Hall, The differential effects of EPA and DHA on cardiovascular risk factors. Proc Nutr Soc, 2011. 70(2): p. 215-31.
58. Egert, S., et al., Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J Nutr, 2009. 139(5): p. 861-8.
59. Jacobson, T.A., et al., Effects of eicosapentaenoic acid and docosahexaenoic acid on low-density lipoprotein cholesterol and other lipids: a review. J Clin Lipidol, 2012. 6(1): p. 5-18.
60. Martins, J.G., EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. J Am Coll Nutr, 2009. 28(5): p. 525-42.
61. Mori, T.A., et al., Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr, 2000. 71(5): p. 1085-94.
62. Mori, T.A., et al., Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation, 2000. 102(11): p. 1264-9.
63. Mori, T.A. and R.J. Woodman, The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care, 2006. 9(2): p. 95-104.
64. Mozaffarian, D. and J.H. Wu, (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr, 2012. 142(3): p. 614S-625S.
65. Wei, M.Y. and T.A. Jacobson, Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Curr Atheroscler Rep, 2011. 13(6): p. 474-83.
66. Woodman, R.J., et al., Docosahexaenoic acid but not eicosapentaenoic acid increases LDL particle size in treated hypertensive type 2 diabetic patients. Diabetes Care, 2003. 26(1): p. 253.
67. Wang, C., et al., n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr, 2006. 84(1): p. 5-17.
68. Deckelbaum, R.J., n-6 and n-3 Fatty acids and atherosclerosis: ratios or amounts? Arterioscler Thromb Vasc Biol, 2010. 30(12): p. 2325-6.
69. Harris, W.S., The omega-6/omega-3 ratio and cardiovascular disease risk: uses and abuses. Curr Atheroscler Rep, 2006. 8(6): p. 453-9.
70. Willett, W.C., Dietary fats and coronary heart disease. J Intern Med, 2012. 272(1): p. 13-24.
71. Rupp, H., et al., Risk stratification by the “EPA+DHA level” and the “EPA/AA ratio” focus on anti-inflammatory and antiarrhythmogenic effects of long-chain omega-3 fatty acids. Herz, 2004. 29(7): p. 673-85.
72. Ohnishi, H. and Y. Saito, Eicosapentaenoic acid (EPA) reduces cardiovascular events: relationship with the EPA/arachidonic acid ratio. J Atheroscler Thromb, 2013. 20(12): p. 861-77.
73. Hirota, S., et al., Low-dose arachidonic acid intake increases erythrocytes and plasma arachidonic acid in young women. Prostaglandins Leukot Essent Fatty Acids, 2010. 83(2): p. 83-8.
74. Kuehl, F.A., Jr. and R.W. Egan, Prostaglandins, arachidonic acid, and inflammation. Science, 1980. 210(4473): p. 978-84.
75. Lin, Y.H., S. Shah, and N. Salem, Jr., Altered essential fatty acid metabolism and composition in rat liver, plasma, heart and brain after microalgal DHA addition to the diet. J Nutr Biochem, 2011. 22(8): p. 758-65.
76. Burns, T., et al., Effect of omega-3 fatty acid supplementation on the arachidonic acid:eicosapentaenoic acid ratio. Pharmacotherapy, 2007. 27(5): p. 633-8.
77. Amtul, Z., et al., Structural insight into the differential effects of omega-3 and omega-6 fatty acids on the production of Abeta peptides and amyloid plaques. J Biol Chem, 2011. 286(8): p. 6100-7.
78. Lambert, M.P., et al., Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A, 1998. 95(11): p. 6448-53.
79. McLean, C.A., et al., Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol, 1999. 46(6): p. 860-6.
80. St George-Hyslop, P.H., Piecing together Alzheimer’s. Sci Am, 2000. 283(6): p. 76-83.
81. Yurko-Mauro, K., Cognitive and cardiovascular benefits of docosahexaenoic acid in aging and cognitive decline. Curr Alzheimer Res, 2010. 7(3): p. 190-6.
82. Innis, S.M. and J.W. Hansen, Plasma fatty acid responses, metabolic effects, and safety of microalgal and fungal oils rich in arachidonic and docosahexaenoic acids in healthy adults. Am J Clin Nutr, 1996. 64(2): p. 159-67.
83. Innis, S.M., Human milk and formula fatty acids. J Pediatr, 1992. 120(4 Pt 2): p. S56-61.
84. Pischon, T., et al., Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation, 2003. 108(2): p. 155-60.
85. Ferrucci, L., et al., Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab, 2006. 91(2): p. 439-46.
86. Horrobin, D.F., Abnormal membrane concentrations of 20 and 22-carbon essential fatty acids: a common link between risk factors and coronary and peripheral vascular disease? Prostaglandins Leukot Essent Fatty Acids, 1995. 53(6): p. 385-96.
87. Horrobin, D.F., et al., Eicosapentaenoic acid and arachidonic acid: collaboration and not antagonism is the key to biological understanding. Prostaglandins Leukot Essent Fatty Acids, 2002. 66(1): p. 83-90.
88. Bosma-den Boer, M.M., M.L. van Wetten, and L. Pruimboom, Chronic inflammatory diseases are stimulated by current lifestyle: how diet, stress levels and medication prevent our body from recovering. Nutr Metab (Lond), 2012. 9(1): p. 32.
89. Hirai, A., [An epidemiological study on dietary ingestion of eicosapentaenoic acid (EPA) and platelet function in Japanese]. Nihon Naika Gakkai Zasshi, 1985. 74(1): p. 13-20.
90. Hirai, A., et al., Eicosapentaenoic acid and platelet function in Japanese. Lancet, 1980. 2(8204): p. 1132-3.
91. Siguel, E.N. and R.H. Lerman, Altered fatty acid metabolism in patients with angiographically documented coronary artery disease. Metabolism, 1994. 43(8): p. 982-93.
92. Wood, D.A., et al., Adipose tissue and platelet fatty acids and coronary heart disease in Scottish men. Lancet, 1984. 2(8395): p. 117-21.
93. Miettinen, T.A., et al., Fatty-acid composition of serum lipids predicts myocardial infarction. Br Med J (Clin Res Ed), 1982. 285(6347): p. 993-6.
94. Kingsbury, K.J., et al., A comparison of the polyunsaturated fatty acids of the plasma cholesteryl esters and subcutaneous depot fats of atheromatous and normal people. Clin Sci, 1962. 22: p. 161-70.
95. Luostarinen, R., M. Boberg, and T. Saldeen, Fatty acid composition in total phospholipids of human coronary arteries in sudden cardiac death. Atherosclerosis, 1993. 99(2): p. 187-93.
96. de Goede, J., et al., N-6 and N-3 fatty acid cholesteryl esters in relation to fatal CHD in a Dutch adult population: a nested case-control study and meta-analysis. PLoS One, 2013. 8(5): p. e59408.
97. de Oliveira Otto, M.C., et al., Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc, 2013. 2(6): p. e000506.
98. Block, R.C., et al., Omega-6 and trans fatty acids in blood cell membranes: a risk factor for acute coronary syndromes? Am Heart J, 2008. 156(6): p. 1117-23.
99. Park, Y., et al., Erythrocyte fatty acid profiles can predict acute non-fatal myocardial infarction. Br J Nutr, 2009. 102(9): p. 1355-61.
100. Luxwolda, M.F., et al., The relation between the omega-3 index and arachidonic acid is bell shaped: synergistic at low EPA+DHA status and antagonistic at high EPA+DHA status. Prostaglandins Leukot Essent Fatty Acids, 2011. 85(3-4): p. 171-8.
101. von Schacky, C., Omega-3 index and cardiovascular health. Nutrients, 2014. 6(2): p. 799-814.
102. Harris, W.S. and C. Von Schacky, The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med, 2004. 39(1): p. 212-20.
103. Harris, W.S., Omega-3 fatty acids and cardiovascular disease: a case for omega-3 index as a new risk factor. Pharmacol Res, 2007. 55(3): p. 217-23.
104. Smith, G.I., et al., Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr, 2011. 93(2): p. 402-12.
105. Smith, G.I., et al., Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin Sci (Lond), 2011. 121(6): p. 267-78.










I should try a bottle of X factor and take one pill with my tablespoon of fish oil then. Yes?
Great website and great article. I am about to start a taking ArA for 1 or 2 months depending on how it goes. After reading around, I have seen recommendations of avoiding fish oil altogether or dosing it 4+ hours away from any ArA dosing. I see that this contradicts with what you have here. I do not intend on terminating my fish oil supplementation, but do you recommend joint dosing or split? Also, it is my first time taking ArA, should I start with 1g or 1.5g?
Thanks