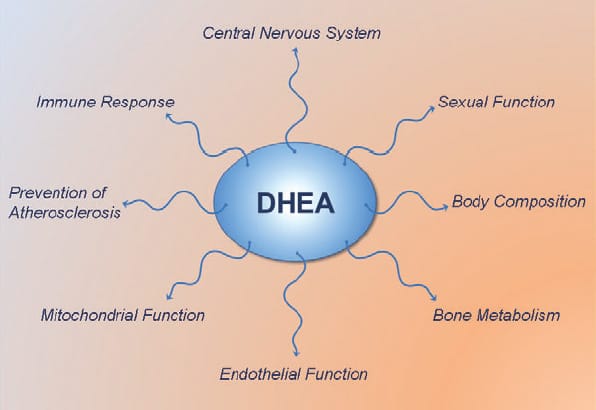
DHEA (dehydroepiandrosterone) is most known for being a pro-hormone which in the body gets converted to testosterone and estrogen. It is a long held view that DHEA exerts all its effects via conversion to testosterone and estrogen. However, recent studies show that DHEA also has several interesting non-hormonal actions…
DHEA 101
DHEA is produced mainly by the adrenal cortex, and is rapidly sulfated by sulfotransferases into DHEA-S. DHEA and its sulfated form DHEA-S is the most abundant steroid (pro)hormone circulating in the blood stream.[1] The sulfated from of DHEA has a longer half-life in the blood and its levels remain stable throughout the day, are not altered significantly by the menstrual cycle. When getting a blood test for DHEA, the fraction that is routinely measured is therefore DHEA-S. In response to metabolic demand, DHEA-S is rapidly hydrolyzed back to DHEA by sulfatases.
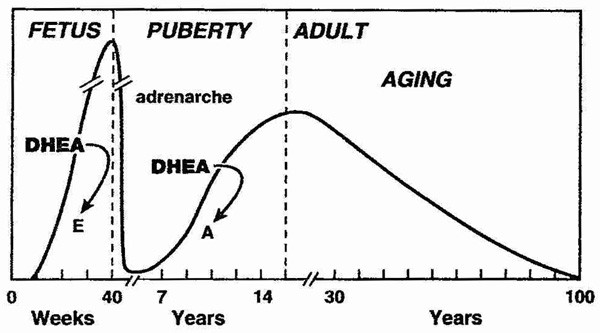
DHEA levels decrease approximately 80% between ages 25 and 75 year.[2, 3] This large decline in DHEA spurred research interest in the possibility that aging related DHEA deficiency may play a role in the deterioration of physiological and metabolic functions with aging, and in the development of chronic diseases.
How does DHEA really work?
No specific cellular nuclear receptor has been identified for DHEA.[4] Therefore, the actions of DHEA have traditionally been thought to be mediated via conversion to testosterone and estradiol, which in turn activate androgen and estrogen receptors and thereby elicits their effects.[5-7] However, emerging research is showing that the action of DHEA also involves multiple other receptors, and that DHEA and /or its oxygenated metabolites, such as epiandrosterone (EpiA) metabolites.[8, 9]
Increased NO production
One DHEA activated receptor, a cell surface (membrane-bound) receptor, that binds DHEA with high affinity has been identified in the endothelium (blood vessel wall), heart, liver, and kidney.[10-13] This receptor is coupled to eNOS (endothelial nitric oxide synthase) [10-12], the enzyme that activates the synthesis of NO (nitric oxide).[14] Endothelial cells exposed to varying concentrations of DHEA produced dose-dependent increased activation of eNOS and elevated nitrate levels (a bi-product of NO).[10, 12] This activation of eNOS by DHEA was not inhibited by the antagonists of the estrogen, androgen, or progesterone receptors, suggesting that eNOS activation by DHEA was through a very specific receptor for DHEA.[12] Support for this comes from another study demonstrating that DHEA supplementation 50 g/day for 2 months in healthy men aged 58+ years increased cGMP (platelet cyclic guanosine-monophosphate) concentrations, which is a marker of NO production.[15]
DHEA also activates an important vascular endothelial cell signaling pathway (ERK1/2) which plays an important role in vascular function.[16] This, combined with the DHEA induced elevation in NO production could explain the beneficial cardiovascular effects which have been seen in several DHEA supplementation studies in humans; such as improvement in vascular endothelial function, arterial stiffness and insulin sensitivity. [17-20]
The DHEA metabolite: 7-beta EpiA
Another exciting finding is that a 7-beta hydroxylated derivative of DHEA is physiologically active via a putative nuclear receptor.[21-23] It is especially notable that 7-beta-hydroxy-epiandrosterone (EpiA) inhibits COX-2 activity (the same enzyme that is targeted by NSAID) and markedly decreases production of the inflammatory prostaglanding PGE(2).[21, 22] 7-beta-EpiA also stimulates production of the anti-inflammatory prostaglandin PGJ(2).[22] Another study confirmed that 7-beta-EpiA stimulates the production of cell protective (cytoprotective) prostaglandins.[23]
These findings have important implications in health and disease. The inflammatory COX-2/PGE2 pathway is an important contributor to the development of cancer, and suppressing the COX-2/PGE2 pathway is a bona fide target for cancer chemoprevention and therapy.[24] In line with this, it has been speculated that a common factor of cancer and the metabolic syndrome may be low DHEA.[25] Thus, there is a good mechanistic basis for a potential beneficial effect of DHEA in inflammatory conditions and for cancer prevention.
Reduced production of pro-inflammatory cytokines and elevated IGF-1 action
Levels of inflammatory markers, such as serum amyloid protein A and C-reactive protein, are inversely correlated with DHEA-S levels, suggesting a role for DHEA in attenuating inordinate inflammatory responses.[26] In addition, DHEA inhibits production of pro-inflammatory cytokines, such as interleukin-6 (IL-6) and TNF-alpha.[20, 27, 28]
As pro-inflammatory cytokines are known to induce IGF-1 resistance [29], by reducing their production DHEA indirectly may improve the responsiveness to IGF-1. DHEA supplementation also increases absolute levels of IGF-1 [30-35] and DHEA metabolites may stimulate aging cells in the anterior pituitary produce growth hormone (called somatotropes).[36] The stimulatory effect of DHEA on the GH/IGF-1 axis in humans is supported by several DHEA supplementation studies.[32-34, 37]
Reduced cortisol levels
DHEA supplementation lowers cortisol levels, even in young adults.[38-42] While it is still unknown exactly how DHEA exerts its anti-cortisol effect, several mechanisms have been proposed.[43] One speculation is that DHEA down regulates glucocorticoid receptors.[44, 45]
Chronically elevated cortisol levels stimulate development of obesity, diabetes, heart disease, mood disorders and memory impairments.[46-53] Administration of DHEA has been demonstrated to counteract the detrimental effects of cortisol in animals [54-56], and in humans, DHEA and cortisol produce opposing effects on the innate immune system; DHEA enhances while cortisol suppresses immunity.[57]
Lowering of cortisol levels with DHEA supplementation can indirectly provide additional benefits. Cortisol breaks down muscle tissue [58, 59], and suppresses testicular testosterone synthesis via multiple mechanisms. Notably, cortisol exerts a direct inhibitory action on testosterone producing Leydig cells in the testicles [60]. Cortisol also induces leptin resistance [53, 61] and thus may diminish leptin’s stimulatory effects on gonadotropin secretion of LH and FSH [62-64] and consequently diminish the secretion of sex hormones from testicles (testosterone) and the ovary (estrogen). Thus, by lowering cortisol levels, DHEA indirectly might confer several additional beneficial effects.
Improved Cortisol/DHEA ratio
DHEA, by reducing blood cortisol levels and elevating DHEA(S) levels, decreases the cortisol/DHEA ratio, which typically increases with age.[65, 66] This imbalance in the cortisol/DHEA ratio has been demonstrated to possibly contribute to several health derangements.
An increased cortisol:DHEAS ratios may contribute to reduced immunity following physical stress in the elderly[57], and may contribute to the development of cognitive impairment [67, 68] and stress-related psychiatric disorders.[69] The increased ratio of cortisol/DHEA-S is also positively associated with the metabolic syndrome and cancer/all-cause mortality.[70, 71] Thus, improvement of the cortisol/DHEA ratio with DHEA supplementation could help counteract age-related catabolism, metabolic dysfunction and prevent development of chronic disease.
Inhibition of the cortisol amplifier 11beta-HSD1
Cortisol levels in the blood is just one part of the picture. Obese humans and rodents have elevated levels of cortisol inside their fat cells (adipocytes), due to an increased activity of 11beta-HSD1 in adipose tissue.[46, 72-76]
11beta-HSD1 (11beta-hydroxysteroid dehydrogenase type 1) is an enzyme that elevates intracellular cortisol levels, irrespective of circulating cortisol levels in the blood, and thereby amplifies cortisol’s fat accumulating actions.[46, 72, 76]
Cause-effect evidence comes from rodent studies showing that transgenic mouse with elevated 11beta-HSD activity have increased cortisol levels in fat cells and develop obesity, expanded visceral (intra-abdominal) fat stores, high blood glucose (hyperglycaemia), blood lipid abnormalities (dyslipidaemia) and hypertension.[77-79] And conversely, pharmacological inhibition of 11beta-HSD1 effectively induces weight loss in obese mice and enhances insulin sensitivity and lowers blood glucose.[80-83]
An increased 11beta-HSD1 activity not promotes expansion of fat stores (especially in the abdominal region) but also contributes to metabolic derangement, which is why 11beta-HSD1 inhibition is an emerging therapeutic target for treatment of obesity, metabolic syndrome and type-2 diabetes.[46, 84-88] DHEA also inhibits 11beta-HSD1 activity in muscle cells, which could be an additional mechanism (on top of simply lowering blood cortisol levels) by which DHEA might prevent cortisol induced insulin resistance and muscle catabolism.[89]
Several studies have demonstrated that DHEA inhibits the activity of 11beta-HSD1 in fat cells from both rodents and humans [90-92], and thereby it counteracts cortisol’s fat storing effect [73, 74]. The inhibitory effect of DHEA on 11beta-HSD1 could be a contributing mechanism to the reduction in total body fat mass [93] and abdominal fat that has been seen with DHEA supplementation.[94]
Bottom Line
While many of the effects of DHEA are mediated via conversion to testosterone and estrogen and activation of the androgen and estrogen receptors, the studies outlined here clearly show that DHEA(S) is biologically active in its own right.
DHEA itself acts through specific cell surface receptors to increase eNOS activity and NO production, and contributes to intracellular signaling through activation of several intracellular messengers. DHEA also suppresses many of the detrimental effects of cortisol in muscle and fat tissue, and increases IGF-1 sensitivity and IGF-1 levels. Thus, it is time to re-evaluate the physiological role of DHEA and appreciate its multifaceted health promoting and potential fat loss actions.
For Additional DHEA info, more articles, videos, etc on DHEA on this site can be found HERE
References:
1. Baulieu, E.E., et al., An Adrenal-Secreted “Androgen”: Dehydroisoandrosterone Sulfate. Its Metabolism and a Tentative Generalization on the Metabolism of Other Steroid Conjugates in Man. Recent Prog Horm Res, 1965. 21: p. 411-500.
2. Orentreich, N., et al., Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab, 1984. 59(3): p. 551-5.
3. Orentreich, N., et al., Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab, 1992. 75(4): p. 1002-4.
4. Widstrom, R.L. and J.S. Dillon, Is there a receptor for dehydroepiandrosterone or dehydroepiandrosterone sulfate? Semin Reprod Med, 2004. 22(4): p. 289-98.
5. Engdahl, C., et al., Role of Androgen and Estrogen Receptors for the Action of Dehydroepiandrosterone (DHEA). Endocrinology, 2014: p. en20131561.
6. Corona, G., et al., Dehydroepiandrosterone supplementation in elderly men: a meta-analysis study of placebo-controlled trials. J Clin Endocrinol Metab, 2013. 98(9): p. 3615-26.
7. Sirrs, S.M. and R.A. Bebb, DHEA: panacea or snake oil? Can Fam Physician, 1999. 45: p. 1723-8.
8. Webb, S.J., et al., The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metab Rev, 2006. 38(1-2): p. 89-116.
9. El Kihel, L., Oxidative metabolism of dehydroepiandrosterone (DHEA) and biologically active oxygenated metabolites of DHEA and epiandrosterone (EpiA)–recent reports. Steroids, 2012. 77(1-2): p. 10-26.
10. Liu, D. and J.S. Dillon, Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Galpha(i2,3). J Biol Chem, 2002. 277(24): p. 21379-88.
11. Liu, D. and J.S. Dillon, Dehydroepiandrosterone stimulates nitric oxide release in vascular endothelial cells: evidence for a cell surface receptor. Steroids, 2004. 69(4): p. 279-89.
12. Simoncini, T., et al., Dehydroepiandrosterone modulates endothelial nitric oxide synthesis via direct genomic and nongenomic mechanisms. Endocrinology, 2003. 144(8): p. 3449-55.
13. Komesaroff, P.A., Unravelling the enigma of dehydroepiandrosterone: moving forward step by step. Endocrinology, 2008. 149(3): p. 886-8.
14. Duckles, S.P. and V.M. Miller, Hormonal modulation of endothelial NO production. Pflugers Arch, 2010. 459(6): p. 841-51.
15. Martina, V., et al., Short-term dehydroepiandrosterone treatment increases platelet cGMP production in elderly male subjects. Clin Endocrinol (Oxf), 2006. 64(3): p. 260-4.
16. Liu, D., et al., Dehydroepiandrosterone stimulates endothelial proliferation and angiogenesis through extracellular signal-regulated kinase 1/2-mediated mechanisms. Endocrinology, 2008. 149(3): p. 889-98.
17. Kawano, H., et al., Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab, 2003. 88(7): p. 3190-5.
18. Weiss, E.P., et al., Dehydroepiandrosterone replacement therapy in older adults improves indices of arterial stiffness. Aging Cell, 2012. 11(5): p. 876-84.
19. Petrie, J.R., et al., Endothelial nitric oxide production and insulin sensitivity. A physiological link with implications for pathogenesis of cardiovascular disease. Circulation, 1996. 93(7): p. 1331-3.
20. Weiss, E.P., et al., Dehydroepiandrosterone (DHEA) replacement decreases insulin resistance and lowers inflammatory cytokines in aging humans. Aging (Albany NY), 2011. 3(5): p. 533-42.
21. Le Mee, S., et al., 7beta-Hydroxy-epiandrosterone-mediated regulation of the prostaglandin synthesis pathway in human peripheral blood monocytes. Steroids, 2008. 73(11): p. 1148-59.
22. Hennebert, O., et al., Anti-inflammatory effects and changes in prostaglandin patterns induced by 7beta-hydroxy-epiandrosterone in rats with colitis. J Steroid Biochem Mol Biol, 2008. 110(3-5): p. 255-62.
23. Davidson, J., E. Wulfert, and D. Rotondo, 7beta-hydroxy-epiandrosterone modulation of 15-deoxy-delta12,14-prostaglandin J2, prostaglandin D2 and prostaglandin E2 production from human mononuclear cells. J Steroid Biochem Mol Biol, 2008. 112(4-5): p. 220-7.
24. Greenhough, A., et al., The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis, 2009. 30(3): p. 377-86.
25. Howard, J.M., Common factor of cancer and the metabolic syndrome may be low DHEA. Ann Epidemiol, 2007. 17(4): p. 270.
26. Sondergaard, H.P., L.O. Hansson, and T. Theorell, The inflammatory markers C-reactive protein and serum amyloid A in refugees with and without posttraumatic stress disorder. Clin Chim Acta, 2004. 342(1-2): p. 93-8.
27. Ramirez, J.A., et al., Syntheses of immunomodulating androstanes and stigmastanes: comparison of their TNF-alpha inhibitory activity. Bioorg Med Chem, 2007. 15(24): p. 7538-44.
28. Straub, R.H., et al., Serum dehydroepiandrosterone (DHEA) and DHEA sulfate are negatively correlated with serum interleukin-6 (IL-6), and DHEA inhibits IL-6 secretion from mononuclear cells in man in vitro: possible link between endocrinosenescence and immunosenescence. J Clin Endocrinol Metab, 1998. 83(6): p. 2012-7.
29. O’Connor, J.C., et al., Regulation of IGF-I function by proinflammatory cytokines: at the interface of immunology and endocrinology. Cell Immunol, 2008. 252(1-2): p. 91-110.
30. Weiss, E.P., et al., Dehydroepiandrosterone replacement therapy in older adults: 1- and 2-y effects on bone. Am J Clin Nutr, 2009. 89(5): p. 1459-67.
31. Igwebuike, A., et al., Lack of dehydroepiandrosterone effect on a combined endurance and resistance exercise program in postmenopausal women. J Clin Endocrinol Metab, 2008. 93(2): p. 534-8.
32. Genazzani, A.D., et al., Oral dehydroepiandrosterone supplementation modulates spontaneous and growth hormone-releasing hormone-induced growth hormone and insulin-like growth factor-1 secretion in early and late postmenopausal women. Fertil Steril, 2001. 76(2): p. 241-8.
33. Morales, A.J., et al., Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab, 1994. 78(6): p. 1360-7.
34. Morales, A.J., et al., The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf), 1998. 49(4): p. 421-32.
35. Villareal, D.T., J.O. Holloszy, and W.M. Kohrt, Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin Endocrinol (Oxf), 2000. 53(5): p. 561-8.
36. Iruthayanathan, M., Y.H. Zhou, and G.V. Childs, Dehydroepiandrosterone restoration of growth hormone gene expression in aging female rats, in vivo and in vitro: evidence for actions via estrogen receptors. Endocrinology, 2005. 146(12): p. 5176-87.
37. Casson, P.R., et al., Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertil Steril, 1998. 70(1): p. 107-10.
38. Genazzani, A.R., et al., Long-term low-dose oral administration of dehydroepiandrosterone modulates adrenal response to adrenocorticotropic hormone in early and late postmenopausal women. Gynecol Endocrinol, 2006. 22(11): p. 627-35.
39. Kroboth, P.D., et al., Influence of DHEA administration on 24-hour cortisol concentrations. J Clin Psychopharmacol, 2003. 23(1): p. 96-9.
40. Alhaj, H.A., A.E. Massey, and R.H. McAllister-Williams, Effects of DHEA administration on episodic memory, cortisol and mood in healthy young men: a double-blind, placebo-controlled study. Psychopharmacology (Berl), 2006. 188(4): p. 541-51.
41. Stomati, M., et al., Six-month oral dehydroepiandrosterone supplementation in early and late postmenopause. Gynecol Endocrinol, 2000. 14(5): p. 342-63.
42. McQuade, R. and A.H. Young, Future therapeutic targets in mood disorders: the glucocorticoid receptor. Br J Psychiatry, 2000. 177: p. 390-5.
43. Kalimi, M., et al., Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Mol Cell Biochem, 1994. 131(2): p. 99-104.
44. Kalimi, M., J. Opoku, and R.e.a. Lu, Studies of the biochemical action and mechanism of dehydroepiandrosterone. , in The biologic role of dehydroepiandrosterone, M. Kalimi and W. Regelson, Editors. 1990: Walter de Gruyter, New York,. p. pp 397-404.
45. Crudo, D., Downregulation of glucocorticoid receptors by dehydroepiandrosterone. 71st Annual Meeting of the Endocrine Society, Seattle, WA, p 386 (Abst), 1989.
46. Wamil, M. and J.R. Seckl, Inhibition of 11beta-hydroxysteroid dehydrogenase type 1 as a promising therapeutic target. Drug Discov Today, 2007. 12(13-14): p. 504-20.
47. Stimson, R.H. and B.R. Walker, Glucocorticoids and 11beta-hydroxysteroid dehydrogenase type 1 in obesity and the metabolic syndrome. Minerva Endocrinol, 2007. 32(3): p. 141-59.
48. Viau, V., Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol, 2002. 14(6): p. 506-13.
49. Pasquali, R., et al., The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann N Y Acad Sci, 2006. 1083: p. 111-28.
50. Boscaro, M., G. Giacchetti, and V. Ronconi, Visceral adipose tissue: emerging role of gluco- and mineralocorticoid hormones in the setting of cardiometabolic alterations. Ann N Y Acad Sci, 2012. 1264: p. 87-102.
51. Dallman, M.F., et al., Minireview: glucocorticoids–food intake, abdominal obesity, and wealthy nations in 2004. Endocrinology, 2004. 145(6): p. 2633-8.
52. Fraser, R., et al., Cortisol effects on body mass, blood pressure, and cholesterol in the general population. Hypertension, 1999. 33(6): p. 1364-8.
53. Ur, E., A. Grossman, and J.P. Despres, Obesity results as a consequence of glucocorticoid induced leptin resistance. Horm Metab Res, 1996. 28(12): p. 744-7.
54. Daynes, R.A., D.J. Dudley, and B.A. Araneo, Regulation of murine lymphokine production in vivo. II. Dehydroepiandrosterone is a natural enhancer of interleukin 2 synthesis by helper T cells. Eur J Immunol, 1990. 20(4): p. 793-802.
55. Daynes, R.A., et al., Regulation of murine lymphokine production in vivo. III. The lymphoid tissue microenvironment exerts regulatory influences over T helper cell function. J Exp Med, 1990. 171(4): p. 979-96.
56. Browne, E.S., et al., Dehydroepiandrosterone: antiglucocorticoid action in mice. Am J Med Sci, 1992. 303(6): p. 366-71.
57. Butcher, S.K., et al., Raised cortisol:DHEAS ratios in the elderly after injury: potential impact upon neutrophil function and immunity. Aging Cell, 2005. 4(6): p. 319-24.
58. Lofberg, E., et al., Effects of high doses of glucocorticoids on free amino acids, ribosomes and protein turnover in human muscle. Eur J Clin Invest, 2002. 32(5): p. 345-53.
59. Rooyackers, O.E. and K.S. Nair, Hormonal regulation of human muscle protein metabolism. Annu Rev Nutr, 1997. 17: p. 457-85.
60. Dong, Q., et al., Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J Androl, 2004. 25(6): p. 973-81.
61. Zakrzewska, K.E., et al., Glucocorticoids as counterregulatory hormones of leptin: toward an understanding of leptin resistance. Diabetes, 1997. 46(4): p. 717-9.
62. Barb, C.R., J.B. Barrett, and R.R. Kraeling, Role of leptin in modulating the hypothalamic-pituitary axis and luteinizing hormone secretion in the prepuberal gilt. Domest Anim Endocrinol, 2004. 26(3): p. 201-14.
63. Yu, W.H., et al., Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci U S A, 1997. 94(3): p. 1023-8.
64. Zieba, D.A., M. Amstalden, and G.L. Williams, Regulatory roles of leptin in reproduction and metabolism: a comparative review. Domest Anim Endocrinol, 2005. 29(1): p. 166-85.
65. Otte, C., et al., A meta-analysis of cortisol response to challenge in human aging: importance of gender. Psychoneuroendocrinology, 2005. 30(1): p. 80-91.
66. Stein-Behrens, B.A. and R.M. Sapolsky, Stress, glucocorticoids, and aging. Aging (Milano), 1992. 4(3): p. 197-210.
67. Kalmijn, S., et al., A prospective study on cortisol, dehydroepiandrosterone sulfate, and cognitive function in the elderly. J Clin Endocrinol Metab, 1998. 83(10): p. 3487-92.
68. Lupien, S.J., et al., Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci, 1998. 1(1): p. 69-73.
69. Garner, B., et al., Cortisol and dehydroepiandrosterone-sulphate levels correlate with symptom severity in first-episode psychosis. J Psychiatr Res, 2011. 45(2): p. 249-55.
70. Phillips, A.C., et al., Cortisol, DHEA sulphate, their ratio, and all-cause and cause-specific mortality in the Vietnam Experience Study. Eur J Endocrinol, 2010. 163(2): p. 285-92.
71. Phillips, A.C., et al., Cortisol, DHEAS, their ratio and the metabolic syndrome: evidence from the Vietnam Experience Study. Eur J Endocrinol, 2010. 162(5): p. 919-23.
72. Walker, B.R., Extra-adrenal regeneration of glucocorticoids by 11beta-hydroxysteroid dehydrogenase type 1: physiological regulator and pharmacological target for energy partitioning. Proc Nutr Soc, 2007. 66(1): p. 1-8.
73. Rask, E., et al., Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab, 2001. 86(3): p. 1418-21.
74. Rask, E., et al., Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11beta-hydroxysteroid dehydrogenase type 1 activity. J Clin Endocrinol Metab, 2002. 87(7): p. 3330-6.
75. Lindsay, R.S., et al., Subcutaneous adipose 11 beta-hydroxysteroid dehydrogenase type 1 activity and messenger ribonucleic acid levels are associated with adiposity and insulinemia in Pima Indians and Caucasians. J Clin Endocrinol Metab, 2003. 88(6): p. 2738-44.
76. Wake, D.J., et al., Local and systemic impact of transcriptional up-regulation of 11beta-hydroxysteroid dehydrogenase type 1 in adipose tissue in human obesity. J Clin Endocrinol Metab, 2003. 88(8): p. 3983-8.
77. Agarwal, A.K., Cortisol metabolism and visceral obesity: role of 11beta-hydroxysteroid dehydrogenase type I enzyme and reduced co-factor NADPH. Endocr Res, 2003. 29(4): p. 411-8.
78. Masuzaki, H., et al., A transgenic model of visceral obesity and the metabolic syndrome. Science, 2001. 294(5549): p. 2166-70.
79. Masuzaki, H., et al., Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice. J Clin Invest, 2003. 112(1): p. 83-90.
80. Hermanowski-Vosatka, A., et al., 11beta-HSD1 inhibition ameliorates metabolic syndrome and prevents progression of atherosclerosis in mice. J Exp Med, 2005. 202(4): p. 517-27.
81. Park, J.S., et al., Anti-diabetic and anti-adipogenic effects of a novel selective 11beta-hydroxysteroid dehydrogenase type 1 inhibitor in the diet-induced obese mice. Eur J Pharmacol, 2012. 691(1-3): p. 19-27.
82. Alberts, P., et al., Selective inhibition of 11beta-hydroxysteroid dehydrogenase type 1 decreases blood glucose concentrations in hyperglycaemic mice. Diabetologia, 2002. 45(11): p. 1528-32.
83. Alberts, P., et al., Selective inhibition of 11 beta-hydroxysteroid dehydrogenase type 1 improves hepatic insulin sensitivity in hyperglycemic mice strains. Endocrinology, 2003. 144(11): p. 4755-62.
84. Anagnostis, P., et al., 11beta-Hydroxysteroid dehydrogenase type 1 inhibitors: novel agents for the treatment of metabolic syndrome and obesity-related disorders? Metabolism, 2013. 62(1): p. 21-33.
85. Tomlinson, J.W., 11Beta-hydroxysteroid dehydrogenase type 1 in human disease: a novel therapeutic target. Minerva Endocrinol, 2005. 30(1): p. 37-46.
86. Morton, N.M. and J.R. Seckl, 11beta-hydroxysteroid dehydrogenase type 1 and obesity. Front Horm Res, 2008. 36: p. 146-64.
87. Joharapurkar, A., et al., 11beta-Hydroxysteroid dehydrogenase type 1: potential therapeutic target for metabolic syndrome. Pharmacol Rep, 2012. 64(5): p. 1055-65.
88. Masuzaki, H. and J.S. Flier, Tissue-specific glucocorticoid reactivating enzyme, 11 beta-hydroxysteroid dehydrogenase type 1 (11 beta-HSD1)–a promising drug target for the treatment of metabolic syndrome. Curr Drug Targets Immune Endocr Metabol Disord, 2003. 3(4): p. 255-62.
89. Whorwood, C.B., et al., Regulation of glucocorticoid receptor alpha and beta isoforms and type I 11beta-hydroxysteroid dehydrogenase expression in human skeletal muscle cells: a key role in the pathogenesis of insulin resistance? J Clin Endocrinol Metab, 2001. 86(5): p. 2296-308.
90. Apostolova, G., et al., Dehydroepiandrosterone inhibits the amplification of glucocorticoid action in adipose tissue. Am J Physiol Endocrinol Metab, 2005. 288(5): p. E957-64.
91. Tagawa, N., et al., Alternative mechanism for anti-obesity effect of dehydroepiandrosterone: possible contribution of 11beta-hydroxysteroid dehydrogenase type 1 inhibition in rodent adipose tissue. Steroids, 2011. 76(14): p. 1546-53.
92. McNelis, J.C., et al., Dehydroepiandrosterone exerts antiglucocorticoid action on human preadipocyte proliferation, differentiation, and glucose uptake. Am J Physiol Endocrinol Metab, 2013. 305(9): p. E1134-44.
93. Libe, R., et al., Effects of dehydroepiandrosterone (DHEA) supplementation on hormonal, metabolic and behavioral status in patients with hypoadrenalism. J Endocrinol Invest, 2004. 27(8): p. 736-41.
94. Villareal, D.T. and J.O. Holloszy, Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA, 2004. 292(18): p. 2243-8.





I have LEF’s DHEA, do I have to put it under my tongue as it says on the bottle? That’s weird….
XD
Most interesting. I have tried DHEA and could never see any response. Perhaps I was taking too little (25 mg PO). LEF seems to recommend putting it under the tongue; does gastric juices inactivate it?
Perhaps Monica could address dose ranges and mode of administration in a future report?
Optimal dose range is decided via blood work as with any hormone. You’ll note it also mentions that on the bottle of LEF DHEA you have. 25mg for a women for actual HRT is on the high end, but unlikely to do you ant harm either.
Like Will said, the optimal dose is dictated by your DHEAS level. If your levels is low, I’d suggest 50-100 mg/day for 2-3 months and then re-test and adjust the dose accordingly.
100 mg DHEA per day is safe, even for prolonged periods if time:
http://www.trainergize.com/diet-supplements/dhea-supplementation-in-older-adults-good-or-bad
It depends on the formulation. You can get it in capsule form as well, which might be preferable.
If your DHEAS level is low-mid rage, 25 mg/day won’t cut it.
Start taking 50 mg/day and go get a blood test for DHEAS; then adjust the dose based on your level.
As a man well beyond my prime this was very interesting, Is there any conjecture as to a possible
dosage level if a person want to try supplementation? Thank you..
Regards,
John
See above comments to Lynn. Blood work is what decides the optimal dosing. Typical HRT dose of DHEA in men is 25-75mg, which is adjusted via responses checked by testing.
As usual Monica you have produced another well researched/documented article.
Phillip, Lyn and John have formulated questions that I too am also interested in.
Specifically what form of DHEA is most easily absorbed? Caps, Tabs, liquid, etc.
As well as determining dose. Does age effect dosage?
Thanks very much for your insights and hard work.
Cliff
Thanks for answer Will…
We must been typing at same time…
Great minds think alike… or so it’s said…
DHEA doesn’t pose any absorption issues like some other supps, so you don’t need any fancy formula. Just get your DHEA supplement from a reputable brand.
Monica, do you have any recommendations on a specific brand of DHEA. I know some have been found to contain no DHEA at all. Thanks, as always, for your excellent and thought-provoking work.
cheers,
mlm
As longs as you stick you reputable brands like eg Life Extension or Vitacost, you have nothing to worry about.
HI Monica,
Again I appreciate the work you do in preparing these articles and the invaluable education it provides.
I have a few comments regarding cortisol.
Cortisol is likely the most misunderstood and important hormone in the human body. If levels drop to 0 we die within 24 hours. It’s primary roles are to regulate glucose, blood pressure and the immune system. It allows us to manage stress and provides drive and dynamism by activating the domaminergic system.
You are absolutely correct that elevated cortisol leads to suppression of the immune system, a catabolic state, psychologic disturbances and various other problems. It’s critically important to also recognize that a cortisol deficiency causes the same problems in addition to chronic fatigue, autoimmune diseases, medication intolerance and various disease states that are diagnosed through a process of exclusion such as irritable bowel syndrome.
Yes, it’s common to have an elevated cortisol/dhea ratio as we age. This is because DHEA levels drop faster than any other hormone. Keep in mind that cortisol also decreases with age. Adding DHEA to a cortisol deficient patient will further inhibit cortisol. Add the sex steroids, growth hormone and melatonin and you have the ideal situation to set up a cortisol deficiency. The result (after a period of time, particularly with athletes) is fatigue, depression, lack of progress and hypothyroidism (deficient cortisol increases T4 to T3 conversion).
As Will indicated, a proper exam, history and lab work is absolutely essential when adding any hormonal supplement. The goal is to achieve a balance between all hormones that are representative of young healthy adults. The problem is that the typical physician will be even more uninformed as it relates to cortisol as compared to the androgens. This is one of the reasons why so many Americans don’t feel right, even after starting a partial hormone replacement program.
Approximately 30 to 50% of middle aged and older adults that seek hormone replacement therapy have at least a mild cortisol deficiency. Chronic hydrocortisone therapy is absolutely safe when a deficiency exists and DHEA as well as the sex steroids are corrected. When done correctly under proper supervision, it will increase athletic performance, body composition, mood/psychological status, energy and decrease degenerative disease.
I always enjoy these articles, and often end at the same point. At seems that the prevailing opinion is that a typical GP or Family Medicine MD may not be well versed in supplementation, what is the best way to get appropriate blood work and assessment/guidance? I’m 51, and like many of my contemporaries, I feel like all the “Low-T” advertising on the radio and in the sports section could be directed right at me (the point of good marketing, I realize). However, when I read more reputable sources (like here), there clearly is a lot of evidence to support the existence of negative issues associated with low T levels.
So, here is my question. I have my annual physical in a few week, with a fasting blood draw being done the week before. Is it appropriate for me to call ahead and request specific assessments be done, and if so what are they? If I ask for T levels, does that provide insight about DHEA levels? Is there values in getting whatever measurements are appropriate, even if my MD may not know what to do with the information (but maybe I could use it for informed decisions about supplements?) Is there a particular MD specialist who would be a more appropriate resource for these types of assessments and supplement decisions?
Thanks for any advice, and for being such a great resource.
No, your T levels won’t tell anything about your DHEA(S) level.
Get this checked (in addition to the standard labs):
total and free T
SHBG
estradiol
DHEAS
IGF-1
advanced lipid panel (VAP or NMR)
PSA
hydroxy-vitamin D
insulin
glucose
HbA1c
CRP
saliva cortisol
Most doctors don’t study nutrition and aren’t practicing preventive medicine, so YES you have to do your homework and be ready to put your foot down and insist on getting the labs I listed.
Yes, the importance of diagnosing and correcting Low-T indeed does have a large body of research backing it up.
While T is classified as a prescription medication, it should be remembered that it is not a foreign chemical to the body like many other medications.
Jim, cortisol responses to different type of stressors increases with age. And the majority suffer health consequences from excessive cortisol levels.
Cortisol, like most all hormones decrease with age. For example, an 80 year old has cortisol levels that are about 75% of a healthy 20 year old.
There are always going to be subgroups that are elevated – for example those that undertake extensive aerobic activity, increase inflammation and believe they are doing something that is improving their health.
Cortisol rapidly changes based on stimulus. The only way to get a true picture of cortisol levels is to collect free and total serum cortisol, cortisol binding globulin and 24 hour urine 17-hydroxy-steroids. The testing must be done under calm, sedentary conditions or it’s not valid.
Jim, you are wrong on that one. Send me studies supporting all your claims and I will be happy to take a look at it.
I loaned the doc my references. I am going to try and get it before the end of the day and look it over this weekend. What could be more exiting on a Valentine’s day than reading cortisol studies. I appreciate your willingness to listen my point of view Monica. I hope you have a great evening.
Jim, I am always open to examine opposing viewpoints, as long as there is good data to support it. You have made several statements I do not agree with, which is why I am asking you for studies to prove your point.
Life Extension has a great article on cortisol you might want to take a look at:
http://www.lef.org/magazine/mag2011/sep2011_Reducing-the-Risks-of-High-Cortisol_01.htm
Hi Monica,
I now have my reference materials back. I have over 350 specific cortisol related studies organized by topic. Since I am not writing an paper here and don’t have a significant amount of space, I will re-summarize my my position. Please note exactly where you disagree and I will try and find an appropriate study or studies.
Please keep in mind that the institutional bias and ignorance does not end with testosterone. Until proven otherwise, it expends to all hormones with the possible exception of insulin. The practicing doc that is ignorant of the endocrine system is trained at the same universities as the researcher conducting the clinical study. Lastly, nothing is ever settled in science. All studies, regardless of quality are of little value if they can’t be validated in clinical practice.
To make this easy I will enumerate my points:
1. Several studies show lower cortisol levels, cortisol metabolites and receptors in the elderly;
2. The highest urinary cortisol metabolites 17-OH-corticoids (levels that reflect the metabolic impact of cortisol) are found in young adults. By the age of 80, urinary metabolites are decreased by 25%.
3. Paradoxically, in other (but not all) studies, higher evening and night serum levels of cortisol have been found in the elderly. This higher serum cortisol and lower urinary metabolites in certain elderly individuals are caused by a reduction in the metabolic clearance with age at target cells, resulting in an accumulation of cortisol in the blood stream.
4. The decrease in intracellular cortisol is aggravated by target cells gradually loosing cortisol receptors with age. This causes a decrease cortisol’s beneficial effects.
5. Regardless of the decrease in beneficial effects, adverse effects from cortisol may appear with age because the decline of anabolic hormones (sex and gh) decline at a much faster rate.
6. An imbalance in favor of catabolism begins and accelerates the aging process in elderly persons unless addressed by corrective hormone therapy.
Great article. Seems to update the old information that played down DHEA and suggested that the use could adversely affect prostate enlargement and cause “gyno”.
Since part of DHEA gets converted to estrogen, it’s important for men to monitor their levels.
Monica: Do you believe that there are any effective supplements that work as estrogen blockers out there on the market?
By the way this is a different Craig than Craig D…so I will go by Craig T for future posts…to follow up on Craig D’s question on tests to ask for…will the results have the ranges posted so that one could know if they are within the “appropriate” ranges? Thank you.
Yes, all labs I know of publish references ranges next to the patient’s values.
A lab’s reference range is just the 95% confidence interval for their reference group. The reference group is not young adults in optimal health, rather it’s skewed to those that are older and/or ill. So, you can land in the lab’s reference range and still be grossly deficient.
This is true for most is not all hormones.
Thee is a study or two that define these reference ranges, however they are based on a symptom list that is generated out of a consensus rather than scientific process.
I agree Monica.
In fact, a trained physician uses lab data only to confirm a diagnosis and catch levels that are grossly out of range. The bulk of the diagnosis is made from clinical findings. Unfortunately, the number of docs that can do this (for the endocrine system) is exceedingly small…
No, you will need to get a script for an aromatase inhibitor from your Dr.
Jim, yes, reference ranges are just that, a reference and not necessarily ideal for everyone. Which is why we need to look at the whole person.
Note that different labs get their reference ranges from different apparently healthy populations.
BTW, I cover (claimed) anti estrogen supplements in my books and programs found here on BZ.
What if you live in a country where Dhea supps arn’t allowed. would 7-keto be an acceptable substitute?
No, 7-keto is yet another metabolite of DHEA, which doesn’t provide the full spectrum of effects that DHEA does.
7-keto is promoted for fat loss; however, the data to support that is relatively weak.
Great Article again 😀 What do you, is this suplemet ideal for my hormonal panel ? (low t, low e2, midle range shbg, high cortisol) I am 23. Posted my blood work in previous articles, but here again. Two years ago, and 2 month ago>>>
(2011)
LH 11,5 + mIU/mL (1.7 – 8.6)
FSH 3.7 mIU/mL (1.5 – 12.4)
Progesterone 0.47 ng/mL (0.20 – 1.40)
Prolactin 317 uIU/ml (86 – 324)
Testosterone 3.46 ng/mL (2.80 – 8.00)
Estradiol 22.84 pg/mL (7.63 – 42.60)
Cortizol 818 nmol/L 8-10 (175-536)
16-20 (64-327)
SHBG 12.0 – nmol/L (14.5 – 48.4)
DHEA-S 339 μg/dL (————-)
TSH 3.280 uIU (0.270 – 4.200)
T3 1.8 nmol/L (1.3 – 3.1)
T4 108 nmol/L (66-181)
………………………………………………………………………………………………..
(2013)
LH 4.6 mIU/mL ( 1.7 – 8.6 )
Testo 2.27- ng/mL (2.80 – 8.00 )
FSH 3.0 mIU/mL ( 1.5 – 12.4 )
SHBG 15.1 nmol/L (14.5 – 48.4 )
Prolactin 406+ uIU/mL ( 86 – 324 )
11:00 AM Prolactin 347+ uIU/mL ( 86 – 324 )
1:00 PM Prolactin 271 uIU/mL ( 86 – 324 )
8:30AM Cortisol 722+ nmol/L (175 – 536 ) ACTH: 32 pg/mL ( 7 – 63 )
4:00PM Cortisol 356+ nmol/L (64 – 327 ) ACTH: 21 pg/mL ( 7 – 63 )
TSH 2.300 uIU/mL (0.270 – 4.200 )
FT4 20 pmol/L (12 – 22 )
blood 11 AM: >>>
LH 9.0+ mIU/mL ( 1.7 – 8.6 )
Testo 1.54– ng/mL (2.80 – 8.00 )
FSH 3.0 mIU/mL ( 1.5 – 12.4 )
SHBG 14.0 nmol/L (14.5 – 48.4 )
Blood 1 PM: >>>>
LH 7.3 mIU/mL ( 1.7 – 8.6 )
Testo 1.70-ng/mL (2.80 – 8.00 )
FSH 3.0 mIU/mL ( 1.5 – 12.4 )
SHBG 13.8 nmol/L (14.5 – 48.4 )
E2 7.49 (7.6 – 42.6) pg/ml
Will dhea lower my cortisol and raise my testosterone and e2 ? Is 50mg a day sufficent for me ?
My endo is not helping me (he thinks everything is ok), so, you people here are my only hope 🙂
If you Endo thinks your levels are fine, get another one!
What jumps at me in your labs is your combination of low-T, high cortisol and mid-range DHEA(S) level.
Here you got DHEA(S) ref ranges: http://www.nlm.nih.gov/medlineplus/ency/article/003717.htm
Therefore yes, you would definitely benefit from DHEA supplementation. Since both your T and E2 is low, and your cortisol is high, I ‘d recommend a higher dose for you, 100 mg DHEA per day until you next blood test.
Again, don’t waste your time with a doctor who is not willing to help you.
A great paper with most needful information. I have started taking DHEA and as with all supplements, have reservations. This most excellent paper answers every question I had and then some. THANK YOU!!!!! Monica rocks!
Thanks for the feedback; I’m thrilled to hear you find my article informative and useful. 🙂
DHEA is something to be used with prudence having used it for many years. Higher pharmacological doses for certain medical conditions should be monitored by a physician with this expertise. Finding a knowledgeable doctor could be a problem in itself. Using physiological doses to keep you in a youthful range should also be monitored by blood tests. One can’t assume where DHEA may cascade into (estrradiol, testosterone). My experience is that if you have no express need for it, don’t use it. Balancing hormones is a juggling act. Although you will read studies using high dose DHEA and proclaiming safety, this is not safe to use long-term for an average user. This is a hormone with a splash effect on other hormones and metabolites.
How do you define “express need for” DHEA?
Like I have said in the past, adding DHEA(S) to your regular blood work exam, together with T and E, should be part of everybody’s health promotion strategy.
And as I have outlined in this article, DHEA is not just about hormones. DHEA has several beneficial effects in its own right. Therefore, I recommend keeping it in the mid-high range. It should be noted thought that there is no one optimal level for everybody. This is why it is so important to do regular blood work as a preventive strategy to establish one’s own baseline and see how different parameters change with different interventions.
Hi Monica,
Have you listed the symptoms associated with DHEA deficiency? The best way to determine “need” is based on physical and psychological symptoms and complaints. Since deficiencies can start in childhood, a baseline may actually indicate long term deficiency and should not be confused with optimal levels for an individual.
One of the first things a physician leans in medical school is to treat the patient and not the lab data. You can’t really fine tune treatment by chasing a lab value. The lab data is collected on a routine basis to verify gross deficiencies and excesses aren’t missed. Once a deficiency is identified, the most appropriate dose is obtained when the symptoms associated with the deficiency are ameliorated. Dosing any higher or lower is outside the optimal range for that individual.
Of course we do know that there are some hormones like testosterone that must be kept above a minimum to avoid degenerative disease, however this level is generally lower than most of us need for optimal health.
I’ve studied DHEA since it came onto the marketplace in 1994. Twenty years later, there is still great disagreement as to why and how it should be used. Having worked within the medical field, it would be accurate to say that the vast majority of medical doctors do not espouse its use. They have enough medical issues to deal with that have greater priority in their eyes. If one decides to use DHEA (or pregnenolone for that matter), find a doctor that is sympathetic and knowledgeable for its use. Have a baseline hormonal panel done as a reference point. You might even find that you don’t need DHEA supplementation since levels vary from person to person. A baseline panel helps a doctor decide on dosage otherwise dosage is done on the blind. Use a DHEA product whose purity can be confirmed. Life Extension Foundation will supply a Certificate of Analysis on their products. For many companies, this is like pulling teeth to get a COA. Expect to do hormone blood testing twice a year and not just DHEA-S alone. A knowledgeable doctor will run at least a basic male or female hormone panel. Whether DHEA actually extends life is debatable, but it may improve quality of life. Keep in mind that many people live long and animated lives without DHEA supplementation. If you decide to use DHEA, there is a commitment to its proper use. Do your own research. Hormones are powerful and need to be used responsibly. If one chooses to use DHEA, use the minimal dosage to meet your needs. Dosage recommendations vary considerably from don’t take it at all, to maximum 5 mg (Dr. Ray Sahelian), and others say higher depending on blood tests, gender, and interpretation of research. If you feel good and are in optimum health, ask yourself why you need to take it. As you research this deeper and deeper, you will find an array of opinions and conflicting recommendations. Give it thought before embarking on its use.
Jim, please email me the cortisol studies you are referring to; when interpreting cortisol data, many factors have to be taken into consideration and results from small studies cannot reliably be extrapolated to the masses.
I would need to type 350 references into an email. Can’t do that.
Tell me specifically where you disagree and I will reply with a few studies.
As an alternate, you could provide studies that show healthy elderly individuals with elevated serum cortisol AND elevated 24 hour urinary metabolites.
I disagree with your statement that cortisol levels decline with age, and that cortisol supplementation is beneficial;
Here you got a well codnucted study showing that free cortisol levels increase with age:
http://press.endocrine.org/doi/pdf/10.1210/jc.2003-030440
LEF has a good article on the detrimental impact of chronically elevated cortisol levels:
http://www.lef.org/magazine/mag2011/sep2011_Reducing-the-Risks-of-High-Cortisol_01.htm?source=search&key=cortisol
When you make claim, at lest add a few links to studies supporting your position.
I want to understand what you are saying first. Are you saying that serum cortisol and 24 hour urinary cortisol metabolites both increase? Without the increase in metabolites, there is no metabolic impact (unless anabolic steroids decrease).
Here is another study showing elevated basal cortisol levels with aging:
http://www.jneurosci.org/content/14/5/2893.long
Now you turn to send me links to studies showing that urinary cortisol metabolites do not increase with age….
I would need to type 350 references into an email. Can’t do that.
Tell me specifically where you disagree and I will reply with a few studies.
As an alternate, you could provide studies that show healthy elderly individuals with elevated serum cortisol AND elevated 24 hour urinary metabolites.
Here are a few:
Moderate decline of cortisol and Senescense:
Age dependent secretion of LH and ACTH in healthy men and patients with erectile dysfunction.
Derouet H1, Lehmann J, Stamm B, Lühl C, Römer D, Georg T, Isenberg E, Gebhardt T, Stoeckle M.
SERUM CORTICOTROPHIN, PLASMA CORTISOL AND URINARY EXCRETION OF 17-KETOGENIC STEROIDS IN THE ELDERLY (AGE GROUP: 66–94 YEARS)
H. Kaalund Jensen and M. Blichert-Toft
Senescence is associated with a lowering of glucocorticoid receptors:
J Steroid Biochem Mol Biol. 1993 Apr;45(1-3):191-4.
Corticosteroid receptors and aging.
Armanini D1, Scali M, Vittadello G, Ribecco M, Zampollo V, Pratesi C, Orlandini E, Zovato S, Zennaro CM, Karbowiak I.
Quality of life and fatigue: association with lower cortisol levels:
Psychoneuroendocrinology. 2009 Nov;34(10):1476-85. doi: 10.1016/j.psyneuen.2009.05.001. Epub 2009 Jun 3.
Cortisol secretion and fatigue: associations in a community based cohort.
Kumari M1, Badrick E, Chandola T, Adam EK, Stafford M, Marmot MG, Kirschbaum C, Kivimaki M.
Heart Disease: Improvement with cortisol treatment:
Crit Care Med. 1975 May-Jun;3(3):94-102.
Effect of methylprednisolone on predicted myocardial infarction size in man.
Morrison J, Maley T, Reduto L, Victa C, Pyros I, Brandon J, Gulotta S.
Obesity:: association with lower cortisol levels:
Am J Physiol Endocrinol Metab. 2007 Aug;293(2):E466-74. Epub 2007 May 15.
Effect of sleep apnea syndrome on the circadian profile of cortisol in obese men.
Dadoun F1, Darmon P, Achard V, Boullu-Ciocca S, Philip-Joet F, Alessi MC, Rey M, Grino M, Dutour A.
Safety of low dose corticosteroids:
Ann Rheum Dis. 2006 Mar;65(3):285-93. Epub 2005 Aug 17.
Safety of low dose glucocorticoid treatment in rheumatoid arthritis: published evidence and prospective trial data.
Da Silva JA1, Jacobs JW, Kirwan JR, Boers M, Saag KG, Inês LB, de Koning EJ, Buttgereit F, Cutolo M, Capell H, Rau R, Bijlsma JW.
urinary cortisol metabolites decrease with age. this is listed below. I have over 50 on just this issue.
SERUM CORTICOTROPHIN, PLASMA CORTISOL AND URINARY EXCRETION OF 17-KETOGENIC STEROIDS IN THE ELDERLY (AGE GROUP: 66–94 YEARS) H. Kaalund Jensen and M. Blichert-Toft – See more at: http://www.brinkzone.com/general-health/dhea-does-it-have-any-beneficial-non-hormonal-effects/#comment-19618
AN INVESTIGATION OF THE URINARY METABOLITES AND SECRETION RATES OF ALDOSTERONE AND CORTISOL IN MAN AND A DESCRIPTION OF METHODS FOR THEIR MEASUREMENT
C. Flood, D. S. Layne, S. Ramcharan, E. Rossipal, J. F. Tait and S. A. S. Tait
Monica, what do you make of the references I sent you showing:
1. Decreased cortisol levels with age;
2. Decreased cortisol urinary metabolites with age; and
3. Benefits of cortisol therapy?
I disagree on #1 and #3.
What urinary cortisol metabolites are you talking about?
Monica,
I’m not sure if you are blowing me off, yanking my chain or are just not interested.
So, the sum total of your response is I don’t agree with 1 and 3 and what cortisol metabolizes are you taking about? Quite frankly I’m not sure what you are taking about??? This is precisely why I haven’t been sending you the studies you are asked for. Why should I bother taking the time to do this if all you are going to do is phone it in?
To be clear I am referring to cortisol urinary metabolite. When cortisol exerts it’s action, it will be metabolized. There is a direct correlation between the effects of cortisol and urinary metabolizes. When serum cortisol rises but urinary cortisol metabolites do not or decrease, that indicated decreased cortisol activity in the target tissues.
I have sent you references showing the above.
I certainly expected more of an explanation as to why you disagree.
Hello Monica, I would like to know which levels of DHEA-S should we get, because you sad to get your level to mid/high range. Is that range specific for every age group, or should we aim to the highest values of 640 ug/dL regardless if someone is 20 or 50 or 70 ?
My DHEAS is 255 ug/dL (24y.o.) , and my fathers is 130 ug/dL (52y.o.). Should both of get our values to near 640 ug/dL ? Thanks
( DHEA(S) ref ranges: http://www.nlm.nih.gov/medlineplus/ency/article/003717.htm – )